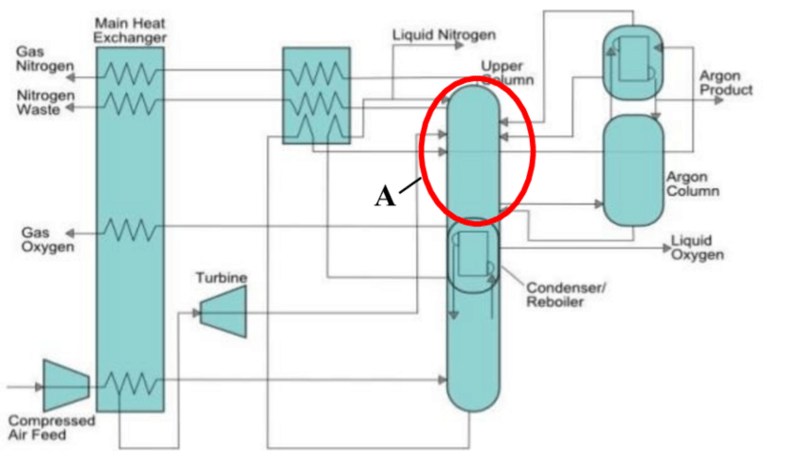
In practice, cryogenic air separation for petrochemical plants must be tightly integrated with downstream units to match pressure, purity and load profiles. Figure: schematic of a cryogenic ASU separating air into its major components. Ambient air is compressed and precooled before entering the distillation section; the main low-pressure column yields liquid O₂ at the bottom and gaseous N₂ at the top, while a side argon column recovers Ar from an intermediate stream.
Cryogenic air separation units (ASUs) are large industrial plants that liquefy and distill ambient air to produce high-purity industrial gases. Through multi-stage compression, CO₂/H₂O removal, deep refrigeration and fractional distillation, they typically deliver oxygen with ≥99.5% purity and nitrogen with ≈99.9% purity, as gaseous or liquid products.
These units are essential in petrochemical complexes, which demand large quantities of pure O₂, N₂ and Ar. Oxygen from ASUs feeds autothermal reformers and partial-oxidation reactors to make synthesis gas for ammonia and methanol, and supplies ethylene-oxide reactors where ethylene is oxidized over silver catalysts. Nitrogen is used extensively for inerting, purging and pressure control. A modern cryogenic ASU may produce from a few hundred up to tens of thousands of Nm³/h of O₂, with co-production of N₂ and Ar.
How Cryogenic ASUs Work
In cryogenic air separation for petrochemical plants, a typical ASU consists of compressors, heat exchangers, distillation columns and storage tanks. Ambient air is first compressed to about 5–10 bar and passed through purification beds to remove moisture and carbon dioxide. The clean compressed air is then cooled in a refrigeration cycle (often using expanded low-pressure product streams and turbo-expanders) down to around –170 °C, where it is partially liquefied before entering the distillation system.
In a standard double-column arrangement, a high-pressure (HP) column feeds a low-pressure (LP) column. Because nitrogen boils at –196 °C and oxygen at –183 °C, nitrogen-rich vapour rises and exits from the top of the HP column, while oxygen-rich liquid sinks to the bottom of the LP column. A side column between the HP and LP sections can withdraw an intermediate stream and refine it to high-purity argon.
This configuration provides continuous streams of gaseous oxygen and nitrogen at the required user pressure (typically 4–10 bar), along with liquid O₂/N₂ when storage is needed. Typical plants achieve oxygen purity of 99.5–99.8% and nitrogen purity around 99.9% or higher. A summary of typical ASU performance is given below:
| Parameter | Cryogenic ASU (Typical) |
|---|---|
| Oxygen purity (gas product) | 99.5–99.8%delion.swiss |
| Nitrogen purity (gas product) | ~99.99% (electronics-grade possible) |
| Cryogenic output capacity (O₂) | ~200 – 40,000+ Nm³/h (up to 120,000 Nm³/h)delion.swissthundersaidenergy.com |
| Cryogenic output capacity (N₂) | ~1,000 – 150,000 Nm³/hthundersaidenergy.com |
| Electricity use | ~0.4–0.8 kWh per Nm³ O₂ (≈400–800 kWh/ton O₂)delion.swissthundersaidenergy.com |
| Typical discharge pressure | ~5–10 bar (pipeline range) |
Most large ASUs deliver both gases at roughly 5–7 bar, suitable for pipeline distribution or direct process use. High-capacity ASUs (on the order of thousands of tons per day of O₂) achieve economies of scale: the larger the plant, the lower the kWh per ton of oxygen producedthundersaidenergy.comdelion.swiss.For long-term projects, cryogenic air separation for petrochemical plants also provides a hedge against volatile liquid-gas prices.

Applications of Cryogenic Air Separation for Petrochemical Plants
Cryogenic air separation for petrochemical plants is deeply integrated into modern petrochemical complexes. Major downstream processes depend on the pure gases from ASUs:
- Syngas and Hydrogen Production (Ammonia/Methanol/Refining): In ammonia and methanol plants, and many hydrogen production units, crude feedstocks (natural gas, naphtha, refinery off-gas or coal derivatives) are partially oxidized with pure O₂ to form synthesis gas. Autothermal reformers (ATRs) and partial-oxidation (POX) reactors require a steady feed of high-purity oxygen to achieve precise H₂/CO ratioseiga.eu. Cryogenic ASUs supply this O₂; for example, an ammonia plant’s gasifier might consume hundreds to thousands of Nm³/h of O₂, compressed to tens of bar. After gasification, cryogenic cryocoolers and columns can further purify the syngas or recover hydrogen from refinery off-gases, since cryogenic separation can efficiently upgrade hydrogen by removing CO₂/CH₄osti.gov.In practice, cryogenic air separation for petrochemical plants is often sized and controlled around these large, continuous syngas and hydrogen production units.
- Ethylene Oxide (EO) Production: Ethylene oxide is produced by the catalytic oxidation of ethylene (from steam crackers) over a silver catalyst. This process uses high-purity O₂ (often produced by ASUs) under controlled pressure (typically 1.5–3 MPa) to oxidize C₂H₄ to C₂H₄Odwsim.fossee.inchemanalyst.com. Precise metering of ASU oxygen is critical for yield and safety: too much O₂ leads to CO₂ by-products, too little reduces EO output. Many EO units are integrated with large ASUs; for example, a gas cracker complex will have a dedicated cryogenic plant that supplies both the EO unit and other chemical processesdwsim.fossee.inchemanalyst.com.This tight coupling of cryogenic air separation for petrochemical plants and EO units ensures stable oxygen supply, high selectivity and safe reactor operation.
- Catalytic Reforming and Hydroprocessing: In naphtha catalytic reforming (to produce high-octane gasoline and hydrogen), the reactor off-gas contains hydrogen plus light hydrocarbons. Although much hydrogen is consumed on-site (for hydrotreating and hydrocracking), surplus H₂-rich gas can be recovered. Cryogenic hydrogen recovery units are used in some refineries/petrochemical plants to purify this H₂: cryogenic separation yields very high-purity H₂ (≥99.99%) and co-produces methane/ethane for fuel, often more economically than PSAosti.gov. In the broader sense, any hydrocarbon hydroprocessing off-gas with hydrogen (e.g. from hydrotreaters or hydrocrackers) can be fed to a cryogenic purifier. Catalytic reforming itself does not use oxygen, but the hydrogen it generates can be captured by cryogenic distillation to improve overall plant efficiencyosti.gov.
- Process Furnaces and Combustion: Many modern crackers, reformers and reactors use oxy-combustion burners (burners with pure O₂ or O₂-enriched air) to achieve higher flame temperatures and lower flue volumes. Cryogenic ASUs can supply oxygen to these heaters, significantly reducing nitrogen in flue gas. For example, a CO₂/CO removing unit or Claus Sulfur plant may burn H₂S-rich tail gas with O₂ from an ASU for sulfur recovery or syngas cleanup.
- Inerting, Purging and Blanketing: Nitrogen is used throughout petrochemical plants to inert equipment, purge pipelines, and maintain safety. ASU-produced N₂ (99%+ purity) provides a reliable source of instrument and process nitrogen. For instance, in polypropylene or polycarbonate plants, liquid nitrogen is used to strip reactors of residual monomers. Storage tanks for volatile feedstocks and products are blanketed with N₂ to prevent air ingress and explosions. Large-scale CO₂ removal units may also use nitrogen to control pressure. Unlike PSA (which serves medium flows), cryogenic N₂ is favored where large, continuous flows of ultra-dry N₂ are needed.When properly sized, cryogenic air separation for petrochemical plants can replace multiple small nitrogen packages with one integrated source of instrument and process nitrogen.
- Argon and Specialty Gases: A cryogenic ASU typically co-produces argon (~0.3–0.5% of output) via the side column. In petrochemical contexts, argon finds niche uses (e.g. as an inert tracer or in specialty polymer processes), but more commonly it is sold commercially.
Technical Advantages and Considerations
Cryogenic air separation for petrochemical plants offers several advantages for large integrated complexes:
- High Purity & Flexibility of Products: Cryogenic ASUs can deliver extremely pure gases: oxygen ≥99.5% and nitrogen ≥99.9%, with the option to adjust purity or produce liquids. They can simultaneously supply multiple gases (O₂, N₂, Ar) and liquids (LOX/LIN) to fit diverse plant needsdelion.swiss.
- Large-Scale Continuous Supply: These units excel in applications with steady, high-volume demand. Large petrochemical complexes with fixed pipelines can justify the high capital cost of cryogenic ASUs. Once running, they provide uninterrupted supply and low marginal cost of gas (especially if integrated with plant energy recovery).
- Thermodynamic Efficiency at Scale: Although power-hungry, cryogenic units become more energy-efficient per unit of gas at higher capacities. Larger ASUs (such as >5,000 Nm³/h) can reach ~0.4 kWh per Nm³ of O₂thundersaidenergy.comdelion.swiss, which translates to ~400 kWh per ton. This is comparable or better than smaller PSA systems at equivalent output. Additionally, improvements like turbo-expander cycles and heat integration reduce the energy footprint.
However, there are also constraints:
- High Capital and Energy Costs: The infrastructure for cryogenic ASUs is complex (cryogenic coldboxes, distillation towers, turbomachinery). CAPEX can be on the order of $200–300 per annual ton of oxygen capacitythundersaidenergy.com, making multi-million-dollar investments. Operating power consumption is significant (often several MW for large plants), and electricity costs dominate OPEXthundersaidenergy.com.
- Inflexibility to Load Changes: Cryogenic ASUs are optimized for steady operation. They have long startup times (hours to days) and limited turndown flexibility. Sudden changes in gas demand (common in some petro runs) can be challenging. Thus they are best suited for plants with baseline loads (e.g., continuous syngas production) and may be paired with small PSA units if intermittent supply is needed.
- Infrastructure Needs: A cryogenic ASU requires substantial footprint (for the cold box and compressors) and on-site utilities (cooling water, purge systems). Bulk O₂ storage (LOX tanks) is common to buffer production and demand.
Despite these considerations, most large petrochemical sites rely on cryogenic units because of their unmatched purity and scale. When well-integrated, the benefits (ensuring pure feedstock gases, improving furnace efficiency, enabling advanced processes) outweigh the costs.

Future Trends and Integration
Cryogenic air separation continues to evolve. Engineers are exploring hybrid approaches (e.g. coupling ASUs with PSA/VPSA or oxygen transport membranes) to improve flexibility or efficiency. In the petrochemical sector, there is a growing interest in using ASU oxygen to reduce carbon footprints (e.g. in gasification of residuals or for new “oxy-fuel” crackers). Likewise, cryogenic units are being adapted to recover and liquefy CO₂ or other byproducts. Nonetheless, the core technology remains the backbone of industrial gas supply. As new petrochem processes emerge (such as bio-derived syngas or power-to-gas with electrolytic O₂), cryogenic ASUs will play a key role in providing high-purity oxygen and nitrogen on-site.
Overall, cryogenic air separation for petrochemical plants enables operators to access the pure gases they need – from multi-ton streams of O₂ for reactors to bulk N₂ for safety – with predictable performance.with predictable performance. By adhering to industry best practices (e.g. co-locating with process plants, optimizing pressure drops, using waste heat), operators can balance the high initial energy draw with long-term reliability. The table above and figures in this article illustrate how cryogenic ASUs deliver critical feedstocks and utilities that drive the modern petrochemical industry.





